If you are having trouble with our mobile app, you must remove and reinstall the app on your device.
Updating the app alone will not fix the issue. Your login will not be impacted. We sincerely apologize for this inconvenience.
Market Pharmacy is now G&G Pharmacy, conveniently located in Marketplace Foods on the corner of Hwy 2 & Broadway!
Same great staff and same great care!
Get Healthy!
- Posted July 18, 2023
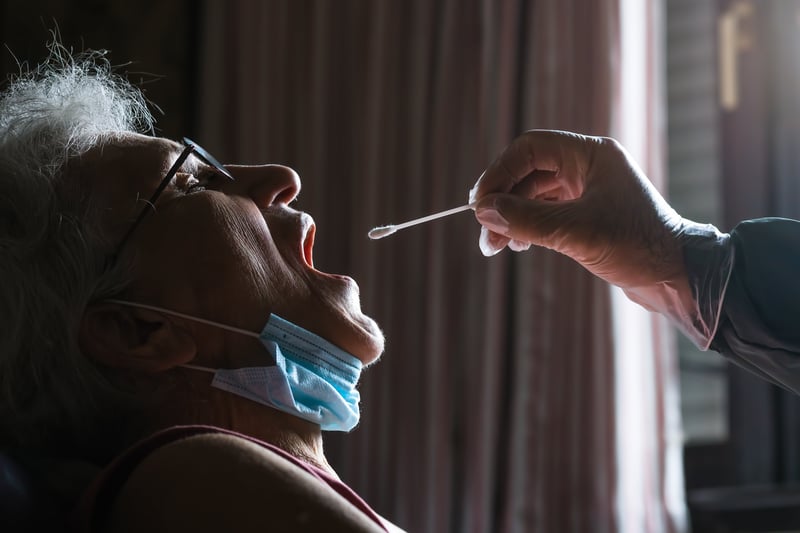
Nursing Homes Used COVID Meds Less Than Expected During Pandemic
While nursing home residents are at high risk for bad outcomes if they get COVID-19, use of antiviral treatments, such as monoclonal antibodies, was low through most of 2021 and 2022.
The authors of a new study, led by Brian McGarry, a health services researcher at the University of Rochester Medical Center in New York, called that fact alarming.
Using data from the U.S. Centers for Disease Control and Prevention National Healthcare Safety Network for May 2021 to December 2002, McGarry and collaborators at Harvard University found that just 18% of COVID-19 cases in nursing homes were treated with antiviral medicine.
Even after easier-to-administer and widely available oral treatments were authorized, only 1 in 4 nursing home residents received these life-saving medications, the investigators found.
The study considered more than 763,000 COVID-19 cases in more than 15,000 U.S. nursing homes. Everyone in a nursing home meets current clinical guidelines to be considered for antiviral treatment.
By the end of last year, however, 40% of nursing homes reported that they had never used any of the antiviral treatments. And for-profit and lower-quality facilities, as well as those with higher shares of Medicaid and non-white residents, were less likely to use antivirals, the study authors noted.
This likely contributed to disparities in COVID hospitalizations and deaths, the researchers suggested in a university news release.
The report was published online July 14 in the Journal of the American Medical Association.
More information
The U.S. Food and Drug Administration has more on antiviral treatment for COVID-19.
SOURCE: University of Rochester Medical Center, news release, July 14, 2023






